Asked by Denise Uruchima on May 27, 2024

Verified
Which of the presented mechanisms would be the most energetically favorable and thus the most likely mechanism to actually occur for the following free radical chain reaction?
(bond dissociation energies - H-H = 104 kcal/mol; Cl-Cl = 58 kcal/mol; H-Cl = 103 kcal/mol)
H2 + Cl2 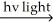 2 HCl
2 HCl
A) H2 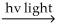 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
H∙ + Cl∙ → HCl
B) Cl2 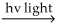 Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl2 → HCl + Cl∙
C) H2 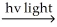 H∙ + H∙
H∙ + H∙
H∙ + Cl2 → Cl∙ + HCl
Cl∙ + H2 → HCl + H∙
D) Cl2  Cl∙ + Cl∙
Cl∙ + Cl∙
Cl∙ + H2 → H∙ + HCl
H∙ + Cl∙ → HCl
Free Radical Chain Reaction
A sequence of reactions where free radicals, generated in an initial step, propagate and react with other molecules to produce stable products, typically involving a chain initiation, propagation, and termination step.
Bond Dissociation Energies
The energy required to break a specific chemical bond in a molecule into two parts, under specified conditions.
- Analyze free radical reactions, including initiation, propagation, and termination steps.

Verified Answer
In option A, the propagation steps involve two exothermic reactions that release a total of 7 kcal/mol of energy. However, the third step involves the recombination of two radicals, which is endothermic and requires 104 kcal/mol of energy, making this mechanism unlikely.
Option C shares the same issue as option A. The last step, involving the recombination of H∙ and Cl∙ to form HCl, is endothermic.
Option D involves a highly endothermic initiation step because it requires breaking the Cl-Cl bond, which has a bond dissociation energy of 58 kcal/mol. The propagation steps release 8 kcal/mol of energy, but the final step requires 104 kcal/mol of energy, making this mechanism energetically unlikely as well.
Option B involves the exothermic formation of chlorine radicals via the homolytic cleavage of the Cl-Cl bond, which releases 58 kcal/mol of energy. The propagation steps involve two exothermic reactions that release a total of 207 kcal/mol of energy. The overall reaction is highly exothermic and yields the desired product. Therefore, option B is the best choice.

Learning Objectives
- Analyze free radical reactions, including initiation, propagation, and termination steps.
Related questions
Does One Expect ΔS° in a Propagation Step of the ...
What Reactive Species Is Produced in the Initiation Step of ...
How Many Distinct Monochlorinated Products Can Result When Cyclopentane Is ...
Which H Atom in the Molecule Shown Will Be Most ...
What Is the Name of the Major Monobrominated Product Which ...