Asked by Cathrina Paris on Jul 31, 2024

Verified
Which of the following terms best describes the stereochemical relationship of the two compounds shown below in Fischer notation? 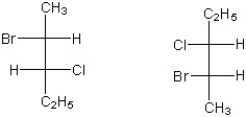
A) enantiomers
B) diastereomers
C) constitutional isomers
D) cis/trans - isomers
E) meso - same structure
Stereochemical Relationship
The relative spatial arrangement of atoms within molecules that dictates their chemical behavior and reactions, focusing on how these relationships affect molecular properties.
Fischer Notation
A method used to represent the three-dimensional structure of molecules using two-dimensional projections, particularly sugars.
Enantiomers
Molecules that are mirror images of each other but cannot be superimposed on each other, often exhibiting different properties in a chiral environment.
- Elucidate the stereochemical linkage between distinct classes of isomers.

Verified Answer

Learning Objectives
- Elucidate the stereochemical linkage between distinct classes of isomers.
Related questions
What Is the Structural Relationship Between the Two Molecule Shown ...
Which of the Following Statements Is (Are) True for the ...
The Relationship Between I and II Is: ________ ...
What Term Describes the Structural Relationship Between (E)- and (Z)-2-Pentene ...
Which of the Following Terms Correctly Describe(s) the Structural Relationship ...