Asked by Chloe Hicks on Jul 31, 2024

Verified
What is the structural relationship between the two molecule shown below? 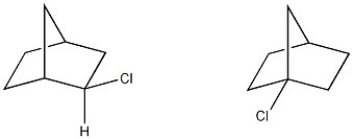
A) constitutional isomers
B) enantiomers
C) diastereomers
D) conformational isomers
E) not isomers
Conformational Isomers
Molecules that have the same molecular formula but differ in the orientation of their atoms in space due to rotation around single bonds.
Constitutional Isomers
Compounds that have the same molecular formula but differ in the connectivity of their atoms, leading to different physical and chemical properties.
Diastereomers
Stereoisomers that are not mirror images of each other, containing two or more stereocenters.
- Comprehend the principle of isomerism and distinguish among different categories of isomers.
- Discern the differences in molecular structure between enantiomers and diastereomers.

Verified Answer

Learning Objectives
- Comprehend the principle of isomerism and distinguish among different categories of isomers.
- Discern the differences in molecular structure between enantiomers and diastereomers.
Related questions
What Term Describes the Structural Relationship Between (2R,3R,4S)-2,3,4-Trichloroheptane and (2S,3S,4R)-2,3,4-Trichloroheptane ...
Glucose and Fructose Are ____ Because They Have Identical Molecular ...
Draw the Structure of (S)-3-Chloro-3-Methylhexane ...
How Many Isomers Can Be Made from the Molecular Formula ...
In an Alkene with Four or More Carbon Atoms in ...