Asked by ??????? ???????? on Jul 12, 2024

Verified
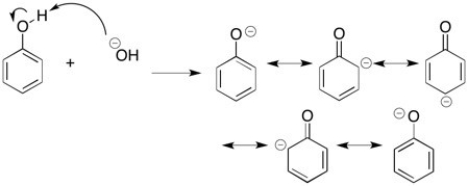
Phenol is more acidic than cyclohexanol because of the resonance stabilization of the phenoxide ion. Draw the proton transfer reaction that take place between sodium hydroxide and phenol. Then show the resonance structures that can be drawn for the phenoxide ion.
Resonance Stabilization
A phenomenon where a molecule can be represented by two or more structures that contribute to its overall stability and properties, enhancing its chemical stability.
Phenoxide Ion
The phenoxide ion is a negatively charged ion (an anion) formed when phenol loses a hydrogen ion (H+), characterized by a phenyl group (C6H5) bound to an oxygen atom carrying a negative charge.
Cyclohexanol
A secondary alcohol with the formula C6H11OH, characterized by a six-member ring structure, and used as a precursor in the production of nylon.
- Understand the concept of acidity in organic compounds and how it relates to resonance stabilization.

Verified Answer

Learning Objectives
- Understand the concept of acidity in organic compounds and how it relates to resonance stabilization.
Related questions
Nitroamines Are Common Functional Groups Found in Energetic Materials, Such ...
When a Negatively Charged Species Is Most Appropriately Depicted as ...
When Acetaldehyde (CH 3 CHO) Is Deprotonated, the Resulting Anion Is Stabilized ...
Provide the Major Resonance Structures of the Ion Which Results ...
Draw the Two Major Resonance Structures of the Acylium Ion ...