Asked by D_Vonte Price on May 16, 2024

Verified
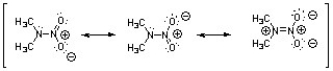
Nitroamines are common functional groups found in energetic materials, such as RDX and HMX. For the structure below, draw two other significant resonance structures, include any formal charges, and indicate the hybridization on each nitrogen and oxygen. 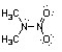
Nitroamines
Organic compounds containing a nitro group (-NO2) bonded to an amine, often explosive.
Resonance Structures
Resonance Structures are alternative Lewis structures for the same molecule, illustrating the delocalization of electrons within the molecule.
Hybridization
A concept in molecular chemistry describing the combination of atomic orbitals to form new hybrid orbitals suitable for the pairing of electrons to form chemical bonds.
- Design exact Lewis structures for molecules and ions, including any applicable resonance structures.
- Recognize and describe the significance of resonance in the stability of molecules.
- Utilize the concept of hybridization to describe the bonding in complex molecules.

Verified Answer

Learning Objectives
- Design exact Lewis structures for molecules and ions, including any applicable resonance structures.
- Recognize and describe the significance of resonance in the stability of molecules.
- Utilize the concept of hybridization to describe the bonding in complex molecules.
Related questions
When a Negatively Charged Species Is Most Appropriately Depicted as ...
Provide the Major Resonance Structures of the Ion Which Results ...
Provide the Hybridization of Oxygen in Dimethyl Ether (CH 3 OCH 3 ) and ...
How Many Resonance Structures Can Be Drawn for Methane ...
Draw the Two Major Resonance Structures of the Acylium Ion ...