Asked by Alexandria Thompson on Apr 23, 2024

Verified
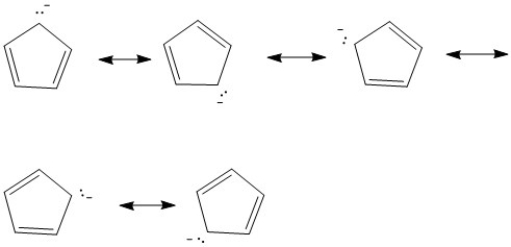
Provide the major resonance structures of the ion which results when the most acidic hydrogen of cyclopentadiene is lost.
Resonance Structures
Different Lewis structures that represent the same molecule, used to depict delocalized electrons within certain compounds.
Cyclopentadiene
A cyclic hydrocarbon with the formula C5H6, known for its role in Diels-Alder reactions.
Acidic Hydrogen
A hydrogen atom bonded to an electronegative atom, making it easily released as a proton (H+).
- Explain the stability of compounds based on resonance structures.

Verified Answer
ZK

Learning Objectives
- Explain the stability of compounds based on resonance structures.
Related questions
What Is the Resonance Energy of a System
The Bond Lengths in a Substituted Cyclobutadiene Compounds Were Determined ...
Nitroamines Are Common Functional Groups Found in Energetic Materials, Such ...
When a Negatively Charged Species Is Most Appropriately Depicted as ...
How Many Resonance Structures Can Be Drawn for Methane ...