Asked by tanner fonoti on Apr 27, 2024

Verified
Consider the reaction of A being converted into B at 25°C. If the ΔG° of this reaction is 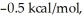 the Keq is ________ and the % conversion is ________.
the Keq is ________ and the % conversion is ________.
A) 0.18; 15%
B) 0.43; 30%
C) 1.0; 50%
D) 2.3; 70%
E) 5.4; 84%
% Conversion
The percentage indicating how much of the reactants have been transformed into products in a chemical reaction.
ΔG°
The change in standard Gibbs free energy during a chemical reaction, indicating spontaneity at standard conditions.
- Explain the function and importance of the equilibrium constant (Keq) within chemical reactions.

Verified Answer
EG
elise greenlandMay 01, 2024
Final Answer :
D
Explanation :
We can use the relationship between ΔG° and K to find the equilibrium constant for the reaction:
ΔG° = -RT lnK
Solving for K, we get:
K = e^(-ΔG°/RT)
Plugging in the given values, we get:
K = e^(-![We can use the relationship between ΔG° and K to find the equilibrium constant for the reaction: ΔG° = -RT lnK Solving for K, we get: K = e^(-ΔG°/RT) Plugging in the given values, we get: K = e^(- /(8.314 J/mol*K * 298 K)) = 2.3 Using the equilibrium constant and the stoichiometry of the reaction, we can find the percentage conversion of A to B: K = [B]/[A] = (x/[A]0)/(1-x), where x is the fraction of A that has reacted and [A]0 is the initial concentration of A. Solving for x, we get: x = K[A]0/(1+K[A]0) = 0.7 Therefore, the % conversion is 70%, which is closest to answer choice D, 2.3; 70%.](https://d2lvgg3v3hfg70.cloudfront.net/TB6199/11eab5e1_c8c5_8be4_9bae_f59b3a2184ad_TB6199_11.jpg) /(8.314 J/mol*K * 298 K)) = 2.3
/(8.314 J/mol*K * 298 K)) = 2.3
Using the equilibrium constant and the stoichiometry of the reaction, we can find the percentage conversion of A to B:
K = [B]/[A] = (x/[A]0)/(1-x), where x is the fraction of A that has reacted and [A]0 is the initial concentration of A.
Solving for x, we get:
x = K[A]0/(1+K[A]0) = 0.7
Therefore, the % conversion is 70%, which is closest to answer choice D, 2.3; 70%.
ΔG° = -RT lnK
Solving for K, we get:
K = e^(-ΔG°/RT)
Plugging in the given values, we get:
K = e^(-
![We can use the relationship between ΔG° and K to find the equilibrium constant for the reaction: ΔG° = -RT lnK Solving for K, we get: K = e^(-ΔG°/RT) Plugging in the given values, we get: K = e^(- /(8.314 J/mol*K * 298 K)) = 2.3 Using the equilibrium constant and the stoichiometry of the reaction, we can find the percentage conversion of A to B: K = [B]/[A] = (x/[A]0)/(1-x), where x is the fraction of A that has reacted and [A]0 is the initial concentration of A. Solving for x, we get: x = K[A]0/(1+K[A]0) = 0.7 Therefore, the % conversion is 70%, which is closest to answer choice D, 2.3; 70%.](https://d2lvgg3v3hfg70.cloudfront.net/TB6199/11eab5e1_c8c5_8be4_9bae_f59b3a2184ad_TB6199_11.jpg) /(8.314 J/mol*K * 298 K)) = 2.3
/(8.314 J/mol*K * 298 K)) = 2.3Using the equilibrium constant and the stoichiometry of the reaction, we can find the percentage conversion of A to B:
K = [B]/[A] = (x/[A]0)/(1-x), where x is the fraction of A that has reacted and [A]0 is the initial concentration of A.
Solving for x, we get:
x = K[A]0/(1+K[A]0) = 0.7
Therefore, the % conversion is 70%, which is closest to answer choice D, 2.3; 70%.

Learning Objectives
- Explain the function and importance of the equilibrium constant (Keq) within chemical reactions.