Asked by Milanpreet kaur Chana on May 25, 2024

Verified
Why can 1,2,2-trimethylaziridine be resolved into enantiomers while 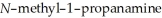
cannot?
Trimethylaziridine
A chemical compound characterized by a three-membered ring structure containing nitrogen and three methyl groups.
Enantiomers
Mirror-image molecules that are non-superimposable, differing in the spatial arrangement of atoms, commonly affecting their chemical behavior in a chiral environment.
- Understand the principles and methods for resolving compounds into enantiomers.

Verified Answer

Learning Objectives
- Understand the principles and methods for resolving compounds into enantiomers.
Related questions
What Is the Structural Relationship Between the Two Molecule Shown ...
What Term Describes the Structural Relationship Between (2R,3R,4S)-2,3,4-Trichloroheptane and (2S,3S,4R)-2,3,4-Trichloroheptane ...
Draw the Structure of (S)-3-Chloro-3-Methylhexane ...
Explain the Stereochemical Relationship, If Any, Among the Following Two ...
Which of the Following Terms Best Describes the Pair of ...