Asked by Joseph Wilson on May 27, 2024

Verified
In the structure below, the sigma bond of the carbonyl is formed from the overlap of a(n) ________ atomic orbital of carbon and a(n) ________ atomic orbital of oxygen. 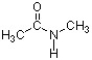
A) sp, sp2
B) sp3, sp2
C) sp2, sp2
D) p, p
Sigma Bond
A type of covalent bond formed by the head-on overlap of atomic orbitals, involving two electrons shared between two atoms.
Atomic Orbital
A mathematical function describing the wave-like behavior of an electron in an atom. These functions define regions in an atom where electrons are most likely to be found.
Carbon
A chemical element with symbol C and atomic number 6, which is the basis of all known life on Earth and a component of many chemical compounds.
- Clarify the notion and criticality of hybrid atomic orbitals in determining the structure of molecules.
- Identify the distinct types of hybridization and assess their effects on molecular structure and angle of bonds.

Verified Answer

Learning Objectives
- Clarify the notion and criticality of hybrid atomic orbitals in determining the structure of molecules.
- Identify the distinct types of hybridization and assess their effects on molecular structure and angle of bonds.
Related questions
What Is the Hybridization of the Positively Charged Carbon in ...
Describe the Hybridization of the Cationic Center and Predict the ...
Provide the Hybridization of Oxygen in Dimethyl Ether (CH 3 OCH 3 ) and ...
From a Molecular Orbital Perspective Why Isn't There Relatively Free ...
Nitroamines Are Common Functional Groups Found in Energetic Materials, Such ...