Asked by Gurlivleen Singh on May 26, 2024

Verified
In the lowest energy conformation of the compound below, how many alkyl substituents are equatorial? 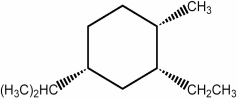
A) 0
B) 1
C) 2
D) 3
E) 6
Alkyl Substituents
Alkyl substituents are groups of atoms, derived from alkanes, that are attached to a molecule, replacing a hydrogen atom.
Equatorial
Refers to the position around a cyclic molecule where substituents are located in the plane or nearly in the plane of the ring, reducing steric hindrance.
Energy Conformation
The spatial arrangement of atoms in a molecule that relates to its energy level, often describing structures that are energetically favorable or unfavorable.
- Examine the stability and location of substituents in chair conformations of substituted cyclohexanes, noting contrasts and comparisons.

Verified Answer

Learning Objectives
- Examine the stability and location of substituents in chair conformations of substituted cyclohexanes, noting contrasts and comparisons.
Related questions
Draw the Most Stable Conformation of Cis-1-Ethyl-4-Isopropylcyclohexane
Draw the Chair Conformation of the Cyclohexane Derivative Shown
Use a Sawhorse Structure to Depict the Eclipsed Conformer of ...
View a Butane Molecule Along the C 2 -C 3 Bond and Provide ...
Consider Rotation About the C3-C4 Bond of Hexane, and Draw ...