Asked by Anette Garcia on Apr 23, 2024

Verified
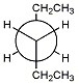
Consider rotation about the C3-C4 bond of hexane, and draw the Newman projection for the most stable conformation.
C3-C4 Bond
In organic chemistry, the covalent bond between the third and fourth carbon atoms in a carbon chain.
Newman Projection
A method of visualizing molecules in chemistry that shows the conformation of a chemical bond from the perspective of looking down the axis of the bond.
Hexane
A saturated hydrocarbon with six carbon atoms and a molecular formula of C6H14.
- Describe and detect distinct arrangements of alkanes by Newman projections and sawhorse configurations.

Verified Answer
SB

Learning Objectives
- Describe and detect distinct arrangements of alkanes by Newman projections and sawhorse configurations.
Related questions
View a Butane Molecule Along the C 2 -C 3 Bond and Provide ...
Use a Sawhorse Structure to Depict the Eclipsed Conformer of ...
Provide a Newman Projection of the Gauche Conformer of Butane
From the Perspective of Viewing Down the C2-C3 Bond, Draw ...
Which of the Statements Below Accurately Describe(s) Alkanes ...