Asked by Miranda Rishi on Jul 31, 2024

Verified
How many moles of carbon dioxide are generated when one mole of the compound shown is treated with warm, concentrated KMnO4? 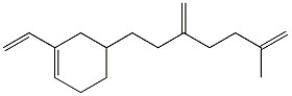
A) 1
B) 2
C) 3
D) 4
E) 8
Compound
A substance made up of atoms of two or more different elements chemically bonded together.
KMnO4
Potassium permanganate, a chemical compound used as an oxidizing agent in various chemical reactions.
- Project the consequences of interactions involving alkenes, with a focus on ozonolysis and additive reactions.

Verified Answer
ZK
Zybrea KnightAug 06, 2024
Final Answer :
C
Explanation :
The balanced chemical equation for the reaction shows that one mole of the compound reacts with 3 moles of KMnO4 to produce 2 moles of CO2. Therefore, when one mole of the compound reacts, 2/3 moles of KMnO4 are consumed, leading to the production of 2/3 moles of CO2. However, since the question asks for the number of moles of CO2 generated, we can round up to the nearest whole number, which is 1.5. This means that when one mole of the compound is treated with KMnO4, approximately 1.5 moles of CO2 are generated. Rounded to the nearest whole number, the answer is 2, which is choice C.

Learning Objectives
- Project the consequences of interactions involving alkenes, with a focus on ozonolysis and additive reactions.
Related questions
A Reaction of an Unknown Alkene with MCPBA in Dichloromethane ...
Give the Structure of the Alkene Which Would Yield the ...
Draw a Chair Conformation of the Starting Substituted Cyclohexane Molecule ...
When Propylene Reacts with Hydrogen Bromide in the Presence of ...
Which of the Following Is the Best Reaction Sequence to ...