Asked by Maria Castro on Jul 31, 2024

Verified
When propylene reacts with hydrogen bromide in the presence of a peroxide initiator, which of the following structures are formed during the mechanism?
A) 
B) 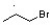
C) 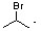
D) H∙
Peroxide Initiator
A compound that decomposes to form free radicals, initiating polymerization reactions.
Propylene
A flammable gas that is a colorless hydrocarbon used as a fuel and a petrochemical feedstock.
- Identify reactants, products, and intermediates in reaction mechanisms involving alkenes.

Verified Answer
FH
Fatin Husna Amat SahriAug 06, 2024
Final Answer :
B
Explanation :
The mechanism for the reaction of propylene with hydrogen bromide in the presence of a peroxide initiator involves the formation of a free radical intermediate. The correct structure formed during the mechanism is 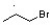 , which shows the free radical intermediate where the bromine has added to the carbon with the most hydrogen atoms. The other structures listed are not involved in the mechanism. H∙ is a hydrogen radical and is not a product of this reaction.
, which shows the free radical intermediate where the bromine has added to the carbon with the most hydrogen atoms. The other structures listed are not involved in the mechanism. H∙ is a hydrogen radical and is not a product of this reaction.
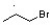 , which shows the free radical intermediate where the bromine has added to the carbon with the most hydrogen atoms. The other structures listed are not involved in the mechanism. H∙ is a hydrogen radical and is not a product of this reaction.
, which shows the free radical intermediate where the bromine has added to the carbon with the most hydrogen atoms. The other structures listed are not involved in the mechanism. H∙ is a hydrogen radical and is not a product of this reaction.
Learning Objectives
- Identify reactants, products, and intermediates in reaction mechanisms involving alkenes.
Related questions
Draw a Chair Conformation of the Starting Substituted Cyclohexane Molecule ...
Which of the Following Intermediates Is Thought to Occur in ...
By What Mechanism Does Cyclohexanol React When Treated in Sulfuric ...
Provide the Reagents Necessary for Carrying Out the Transformation of ...
Which of the Following Alkenes Is the Major Product When ...