Asked by Veronica Otoya-Salazar on May 28, 2024

Verified
Which series of reactions described below will result in the formation of compound A, starting with compound B? 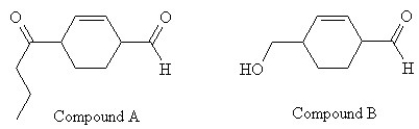
A) 1. HO-(CH2) 2-OH /trace H3O+
2) DMSO (COCl2) /Et3N, CH2Cl2
3) MgBr-(CH2) 2-CH3/diethyl ether
4) work-up with H3O+
5. PCC
B) 1. PCC
2) SOCl2
3) LiCu-((CH2) 2-CH3) 2
4) work-up with H3O+
C) 1. Na2Cr2O7/H2SO4
2) SOCl2
3) 2 MgBr-(CH2) 2-CH3/diethyl ether
4) work-up with H3O+
D) both A and B
E) both B and C
PCC
Pyridinium chlorochromate, a reagent used in organic chemistry for the oxidation of primary alcohols to aldehydes and secondary alcohols to ketones, maintaining the carbon skeleton.
LiCu
Lithium cuprate, a compound used in organic synthesis for coupling reactions.
- Determine the foremost organic product yielded in a range of organic reactions.
- Examine the role and usage of particular reagents in the process of organic synthesis.

Verified Answer
AC
Anita CeballosMay 31, 2024
Final Answer :
A
Explanation :
The formation of compound A involves the following steps:
1. Protection of hydroxyl group with a sulfonate (HO-(CH2)2-OH)
2. Reaction with thionyl chloride and subsequent treatment with DMSO and Et3N leads to formation of the acid chloride (COCl2).
3. Reaction with magnesium bromide and subsequent workup leads to formation of the corresponding Grignard reagent.
4. Reaction with the sulfonate-protected alcohol and work-up with hydrogen peroxide in pyridine leads to the desired product (compound A).
None of the other reaction sequences described would lead to the formation of compound A.
1. Protection of hydroxyl group with a sulfonate (HO-(CH2)2-OH)
2. Reaction with thionyl chloride and subsequent treatment with DMSO and Et3N leads to formation of the acid chloride (COCl2).
3. Reaction with magnesium bromide and subsequent workup leads to formation of the corresponding Grignard reagent.
4. Reaction with the sulfonate-protected alcohol and work-up with hydrogen peroxide in pyridine leads to the desired product (compound A).
None of the other reaction sequences described would lead to the formation of compound A.

Learning Objectives
- Determine the foremost organic product yielded in a range of organic reactions.
- Examine the role and usage of particular reagents in the process of organic synthesis.
Related questions
Provide the Structure of the Major Organic Product Which Results ...
Provide the Major Organic Product of the Reaction Shown Below ...
Provide the Major Organic Product of the Reaction Below
Draw the Major Organic Product(s) Generated in the Reaction Below ...
Provide the Major Organic Product of the Following Reaction