Asked by Colby Raley on Jul 31, 2024

Verified
Which of the following statements correctly describes the contribution of 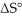 to
to 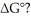
A) The entropy term makes a greater contribution to ΔG° at low temperatures.
B) The entropy term makes a greater contribution to ΔG° at high temperatures.
C) The entropy term makes a greater contribution to ΔG° in exothermic reactions.
D) The entropy term makes a greater contribution to ΔG° in endothermic reactions.
E) The entropy term always makes a more significant contribution to ΔG° than does the enthalpy term.
Entropy Term
A thermodynamic quantity representing the degree of disorder or randomness in a system, significant in predicting the spontaneity of processes.
ΔG°
Standard Gibbs free energy change, a thermodynamic quantity indicating the spontaneity of a reaction at standard conditions; negative values suggest spontaneity.
Exothermic
A chemical reaction or process that releases heat to its surroundings.
- Understand the principles of Gibbs free energy and its components enthalpy and entropy in chemical reactions.

Verified Answer
ZK
Zybrea KnightAug 04, 2024
Final Answer :
B
Explanation :
The given compounds have identical enthalpy (H) values, but they differ in their entropy (S) values. Therefore, the contribution of the entropy term (TΔS) to the overall Gibbs free energy change (ΔG°) increases with increasing temperature. At high temperatures, the entropy term dominates in determining the sign and magnitude of ΔG°. Therefore, choice B is the correct answer.

Learning Objectives
- Understand the principles of Gibbs free energy and its components enthalpy and entropy in chemical reactions.