Asked by Hannah Bretz on Jun 09, 2024

Verified
Which of the following compounds would show only one triplet in its off resonance decoupled 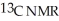 spectrum?
spectrum?
A) acetone
B) butanal
C) pentanal
D) 2-pentanone
E) 3-pentanone
Off Resonance Decoupled
A technique in NMR spectroscopy used to simplify spectral interpretation by selectively decoupling the spins of specific nuclei from their scalar coupling with others.
Triplet
An atomic or molecular state characterized by three possible values of its magnetic quantum number, indicating a level of spin degeneracy.
- Attain an understanding of the key principles of different spectroscopic techniques (NMR, IR, Mass, UV) for the structural determination and characterization of organic molecules.

Verified Answer
ZK
Zybrea KnightJun 10, 2024
Final Answer :
E
Explanation :
In off-resonance decoupling, all proton-proton scalar couplings are removed. As a result, each set of chemically equivalent protons will appear as a single peak in the spectrum. Since the question asks for a compound that shows only one triplet, we need to look for a compound that has only one set of chemically equivalent protons that are coupled to each other with a scalar coupling constant of J.
Acetone has three sets of chemically equivalent protons: the methyl group and the two carbonyl groups. Each set would give a triplet and therefore we would see three triplets in the spectrum.
Similarly, butanal has two sets of chemically equivalent protons: the methyl group and the methylene group. Each set would give a triplet and therefore we would see two triplets in the spectrum.
Pentanal has three sets of chemically equivalent protons: the two methyl groups and the methylene group next to the carbonyl group. Each set would give a triplet and therefore we would see three triplets in the spectrum.
2-pentanone has two sets of chemically equivalent protons: the methyl group and the methylene group next to the carbonyl group. Each set would give a triplet and therefore we would see two triplets in the spectrum.
3-pentanone has only one set of chemically equivalent protons that are coupled to each other with a scalar coupling constant of J. Therefore, we would see only one triplet in the spectrum.
Acetone has three sets of chemically equivalent protons: the methyl group and the two carbonyl groups. Each set would give a triplet and therefore we would see three triplets in the spectrum.
Similarly, butanal has two sets of chemically equivalent protons: the methyl group and the methylene group. Each set would give a triplet and therefore we would see two triplets in the spectrum.
Pentanal has three sets of chemically equivalent protons: the two methyl groups and the methylene group next to the carbonyl group. Each set would give a triplet and therefore we would see three triplets in the spectrum.
2-pentanone has two sets of chemically equivalent protons: the methyl group and the methylene group next to the carbonyl group. Each set would give a triplet and therefore we would see two triplets in the spectrum.
3-pentanone has only one set of chemically equivalent protons that are coupled to each other with a scalar coupling constant of J. Therefore, we would see only one triplet in the spectrum.

Learning Objectives
- Attain an understanding of the key principles of different spectroscopic techniques (NMR, IR, Mass, UV) for the structural determination and characterization of organic molecules.
Related questions
In the Mass Spectrum of 3,3-Dimethyl-2-Butanone, the Base Peak Will ...
How Does the O-H Stretch in the IR Spectrum of ...
Deduce the Identity of the Compound Whose Molecular Formula Is ...
Deduce the Identity of the Following Compound from the 1 H ...
The Chair Form of Cyclohexane Has Protons in Two Distinct ...