Asked by Santiago Talamantes on May 28, 2024

Verified
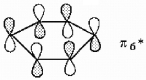
Show how the participating p orbitals interact to form the highest energy π molecular orbital of benzene.
P Orbitals
Atomic orbitals shaped like dumbbells, consisting of regions where there's a high probability of finding an electron outside the nucleus, pivotal in chemical bonding and valence.
Highest Energy
Refers to the state of a system or particle where it possesses the maximum amount of energy compared to other possible states.
- Understand the relationship between molecular orbital theory and the chemical behavior of aromatic compounds.

Verified Answer
AM

Learning Objectives
- Understand the relationship between molecular orbital theory and the chemical behavior of aromatic compounds.
Related questions
From a Molecular Orbital Perspective Why Isn't There Relatively Free ...
For What Does the Acronym HOMO Stand
What Name Is Given to a Hydrocarbon That Contains a ...
Absorption of UV-Visible Energy by a Molecule Results in ________ ...
UV Spectroscopy Measures the Energy Required to Promote an Electron ...