Asked by Jestina Maron on Apr 23, 2024

Verified
Explain why the synthetic route shown below would be unsuccessful. 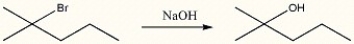
Synthetic Route
The planned sequence of chemical reactions used to convert starting materials into desired products in the laboratory or industry.
- Design and understand synthetic pathways for the transformation of organic compounds.

Verified Answer
ZK
Zybrea KnightMay 02, 2024
Final Answer :
The tertiary bromide is too hindered to undergo an SN2 reaction with hydroxide. However, the hydroxide is a strong base and would react with the bromide above to yield an alkene via an E2 mechanism.

Learning Objectives
- Design and understand synthetic pathways for the transformation of organic compounds.
Related questions
Provide the Reagents Necessary to Accomplish the Following Transformation ...
Provide the Name of the Major Organic Product That Results ...
Provide the Reagents Necessary to Carry Out the Conversion Shown ...
Provide the Name of the Major Organic Product That Results ...
What Series of Synthetic Steps Could Be Used to Prepare ...