Asked by Bailey Hembree on May 27, 2024

Verified
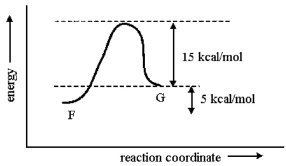
Consider the one-step conversion of F to G. Given that the reaction is endothermic by 5 kcal/mol and that the energy difference between G and the transition state for the process is 15 kcal/mol, sketch a reaction-energy diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.
Endothermic
A reaction or process that absorbs energy from its surroundings, often in the form of heat.
- Evaluate reaction energy diagrams for the purpose of understanding reaction mechanisms and energetics.

Verified Answer
KP

Learning Objectives
- Evaluate reaction energy diagrams for the purpose of understanding reaction mechanisms and energetics.
Related questions
Consider the Three-Step Mechanism for the Reaction of a Through ...
The Following [3+2] Cycloaddition Was Reported in Organic Letters, 2011 ...
Potential Energy Stored in Bonds of Molecules Is ________ Energy ...
The Energy Stored in ATP Is a Form of ________ ...
The Conversion Between Different States of Energy (E ...