Asked by GURPREET SINGH on Jun 03, 2024

Verified
Complete the following acid/base reaction and use pKa or pKb values to predict whether the equilibrium will favor the reactants or products: 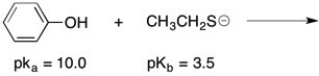
pKa
A quantitative measure that indicates the strength of an acid in solution, specifically the acid's dissociation constant.
pKb
A measure of the basicity of a compound, defined as the negative logarithm of the base dissociation constant (Kb).
Acid/Base Reaction
A chemical reaction that occurs between an acid and a base, often resulting in the formation of a salt and water.
- Execute calculations and provide interpretations for Ka and pKa values.
- Apply acid-base principles to solve chemical equilibrium problems.

Verified Answer
AC
Alexa CarpioJun 04, 2024
Final Answer : 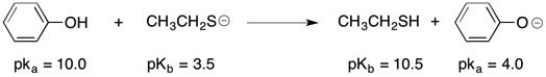 The stronger acid and the stronger base are both on the left side of the reaction (reactants); therefore, the equilibrium concentration should favor the products or right side of this equation.
The stronger acid and the stronger base are both on the left side of the reaction (reactants); therefore, the equilibrium concentration should favor the products or right side of this equation.
 The stronger acid and the stronger base are both on the left side of the reaction (reactants); therefore, the equilibrium concentration should favor the products or right side of this equation.
The stronger acid and the stronger base are both on the left side of the reaction (reactants); therefore, the equilibrium concentration should favor the products or right side of this equation.
Learning Objectives
- Execute calculations and provide interpretations for Ka and pKa values.
- Apply acid-base principles to solve chemical equilibrium problems.