Asked by Chantal Shanty on Apr 23, 2024

Verified
Anthocyanidins are the organic compounds largely responsible for the color of wine and grape juice. They can also be used as indicators in pH titrations. Given the reaction below, which form of the compound is colored and which is colorless. 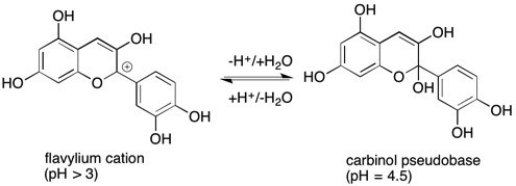
Anthocyanidins
Plant pigments that belong to the flavonoid group, responsible for the red, purple, and blue colors in many fruits and flowers.
pH Titrations
Analytical methods used to determine the concentration of an acid or base in a solution by gradually adding a known concentration of titrant until the reaction reaches its equivalence point, indicated by a pH change.
- Find and describe the structural configurations of primary organic output from a range of organic reactions.
- Analyze and predict the outcomes of cycloaddition reactions, both thermal and photochemical.

Verified Answer
ZK
Zybrea KnightMay 02, 2024
Final Answer :
The carbinal pseudobase form of the anthocyanidin is most likely the colorless form. The extended conjugated network has been disrupted by the bonding of the OH.

Learning Objectives
- Find and describe the structural configurations of primary organic output from a range of organic reactions.
- Analyze and predict the outcomes of cycloaddition reactions, both thermal and photochemical.
Related questions
The Following [3+2] Cycloaddition Was Reported in Organic Letters, 2011 ...
Provide the Major Organic Product of the Following Reaction
Predict the Major Product of the Following Reaction
Provide the Structure of the Major Organic Product in the ...
Provide the Structure of the Major Organic Product Which Results ...